eCTD Submission Services
Speak with an SPharm Advisor
Download Report
Our scope of work includes electronic Common Technical Document (eCTD) publishing services. The most efficient path to regulatory approvals is to have your regulatory expert(s) also manage eCTD submissions. We work in close collaboration with your technical publishing team applying the strictest of regulatory affairs compliance to the publishing process and ensuring the most efficient electronic submission success and successful market access.

Common Technical Document?
The CTD format originates from the International Conference on Harmonization (ICH) initiatives, in their effort to harmonize efficacy, safety and quality (chemistry and manufacturing) requirements globally for the registration of drugs (pharmaceuticals, biologicals, genetic therapies, etc.) for human use. This initiative includes standard information organization for new drug registration applications.
CTD TRIANGLE
The CTD format is divided into five modules: Module 1 contains region-specific information and Modules 2–5 contain common clinical, nonclinical and quality information with some regional variations.
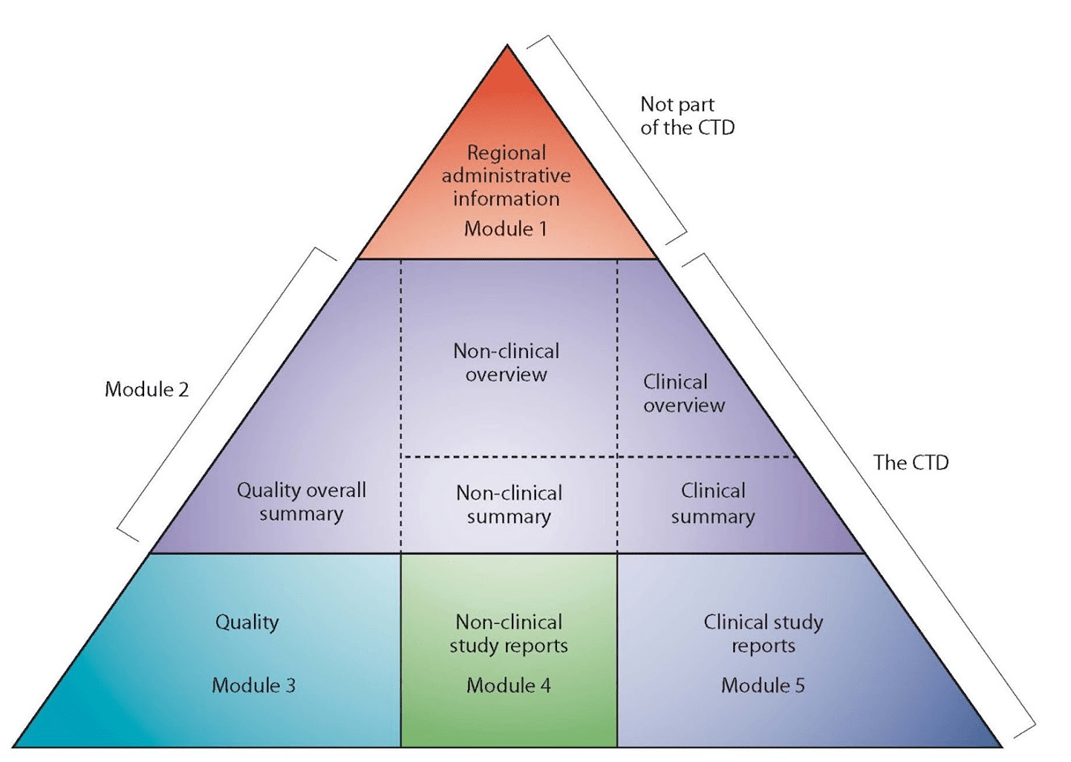
The Module 1 (regional) includes the following, amongst other information:
- Administrative form
- Product Monograph
- Mock-up of Inner and Outer labels
- Certified Product Information Document
- Brand Name Analysis
- Risk Management Plan
- Etc.
We, at SPharm, have earned a proud reputation for being skilled and multidisciplinary professionals, well-known for being responsive and efficient when facing short timelines with high workload and adjusting to client and regulator agency’s needs at all times.
Our Track Record of Success
NUMBER OF SUBMISSIONS
Over 1500 submissions in the past 10 years
SUBMISSION SUCCESS RATE
Near 99%
CLIENT LOCATIONS
SPharm has delivered regulatory services to clients in 15 different countries
A Key Factor in Our Clients' Success



Find out what SPharm Regulatory Consultants can do for you.
Learn how our proven expertise can help reach your objectives faster. Contact us today.
