Canada
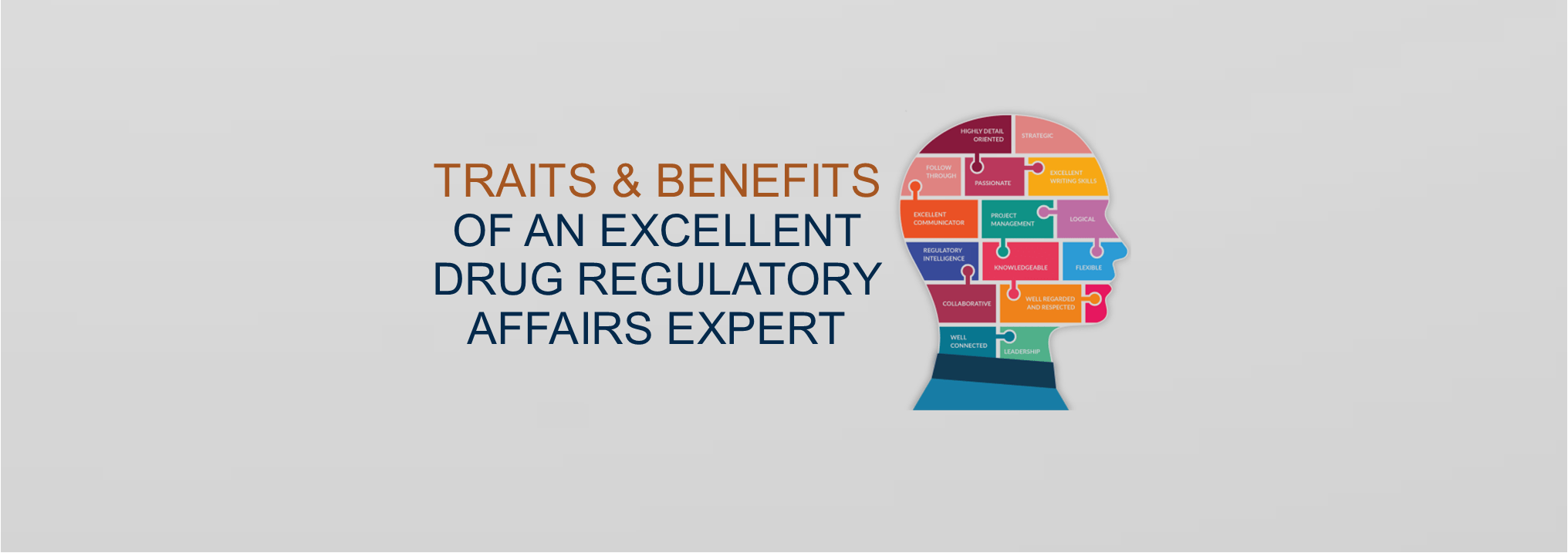
Drug Regulatory Services
Looking for Regulatory Consultants to get approval for your Drugs, Medical Devices and other Products?
Benefit from SPharm’s over 25 years of experience and relationships.
Benefit from SPharm’s over 25 years of experience and relationships.