In Canada, Health Canada has various licensing pathways for drugs, medical devices and other health products and they continue to work towards different pathways like the Priority Review New Drug Submission to facilitate market access of key health products, particularly those for unmet medical needs.
The regulatory strategies and submission work are done by regulatory experts; professionals well-versed and experienced in how each regulatory body evaluates and approves New Drug Submissions (NDS), Medical Devices License Applications as well as other health product applications in Canada or their equivalent outside Canada. This article will focus on what is called Priority Review New Drug Submission Process by Health Canada.
If you are looking to conduct a clinical trial in Canada or submit for the marketing authorization of a new health product, it is important to be familiar with the overall new drug submission process in Canada.
Priority Review New Drug Submission Process in Canada – PR NDS
A Priority Review (PR) New Drug Submission (NDS) is a type of regulatory filing submitted to Health Canada for the review of a new drug, at the end of its clinical development program, that is considered to have the potential to provide significant benefit over existing therapies (when available) for serious or life-threatening conditions. PR NDSs are prioritized over standard NDSs and are scientifically reviewed within 180 days, rather than the standard review time of 300 days. This expedited review process is intended to help bring new, innovative treatments to patients in need more quickly.
To apply for a Priority Review NDS, a sponsor must demonstrate that the drug is to be used for a serious, life-threatening, or severely debilitating condition when there is substantial evidence of clinical effectiveness that shows the following:
- effective treatment, prevention or diagnosis of a disease, for which no therapy is available in Canada, or
- significant increase of efficacy and/or decrease in risk, supporting an improved benefit/risk profile over available treatment, prevention, or diagnostic agent for a disease, not adequately managed by available agents in Canada.
In summary, Priority Review New Drug Submission Process in Canada allows for drugs with a complete clinical program that address important unmet medical needs. The sponsor has to apply for a PR through an official request supported by a Clinical Assessment Package, justifying scientifically and with sufficient data why the submission should be granted PR rather than following the standard NDS process.
The Priority Review process differs from the regular NDS process as presented below:
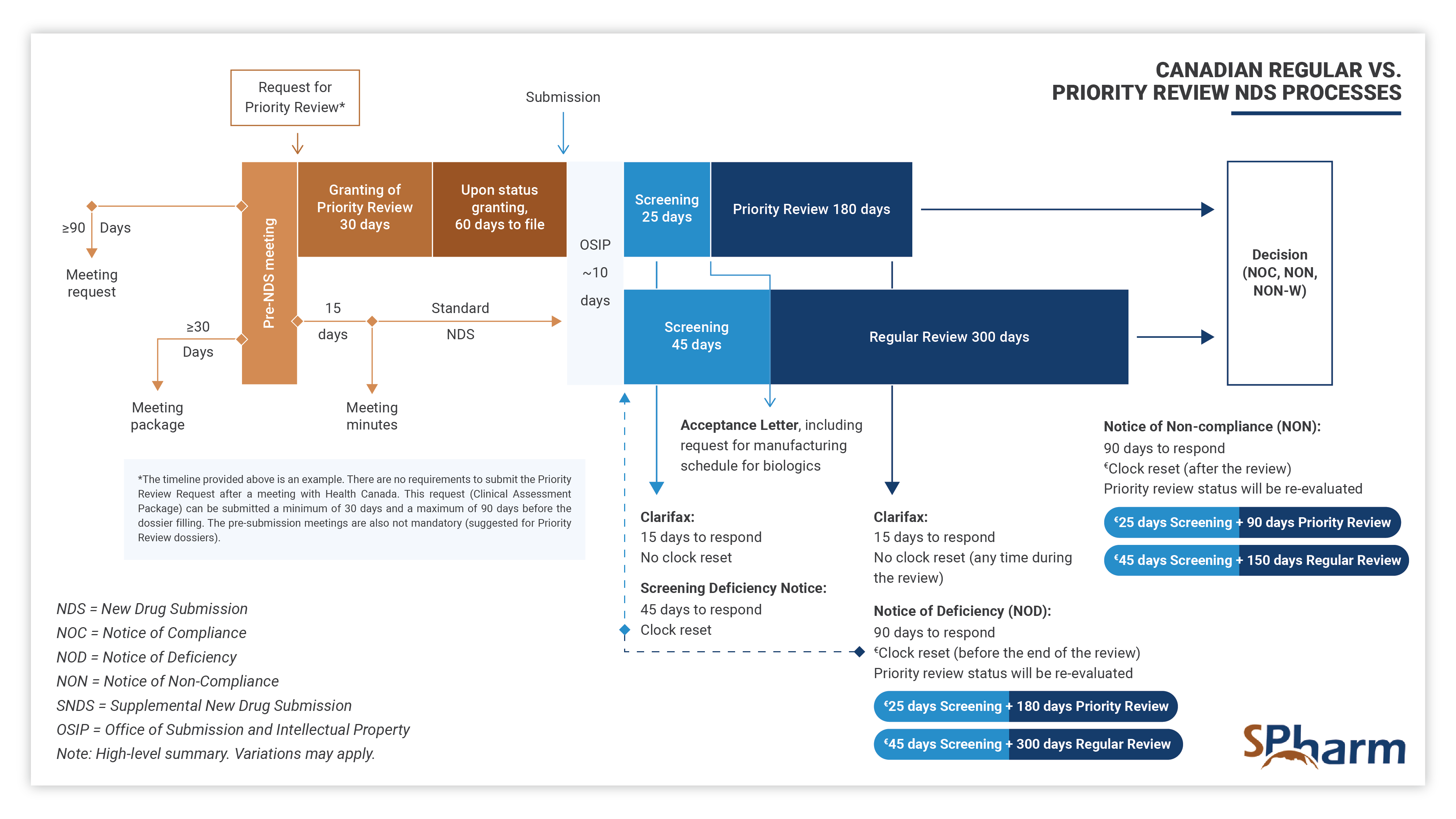
After the Notice of Compliance – NOC
Once Health has reviewed the submission and determined that the drug is safe and effective for its population, they will issue a Notice of Compliance (NOC), which grants the sponsor permission to sell the drug in Canada.
The Role of Regulatory Experts in Canada
It is the role of regulatory experts to strategize with you about which pathway to follow, and to navigate these applications through Health Canada or their equivalent outside of Canada, to liaise with regulatory body officials, and lead the submission process.
FURTHER INFORMATION
For further information about the drug review & approval process in Canada, or about the New Drug Submission Process with Health Canada or to have a complementary discussion about your needs, please contact SPharm directly.